Pure Component Properties
Understanding what's makes up your C7+ components is important, and you shouldn't need to have a PhD in chemistry to have access to this knowledge 👨🏫! This section is meant to be a guide to get you up-to-speed on the basics of thermochemistry for hydrocarbon compunds!
Laboratory Component Slate
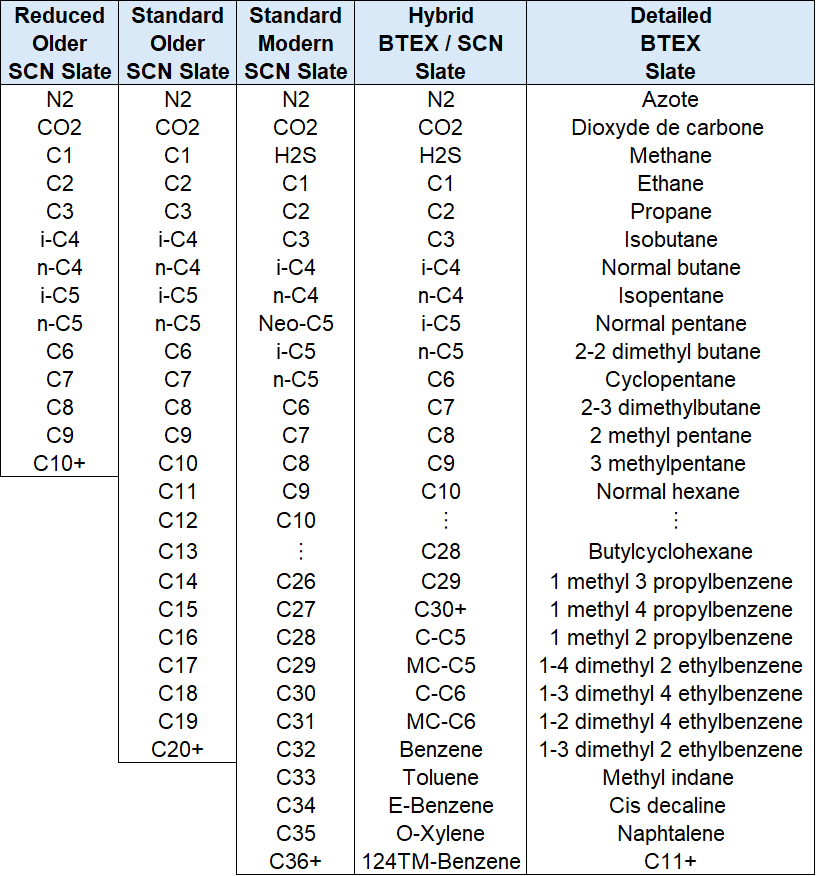
In PVT reports, there are many different variations of how the compositional data is reported! Some different examples are shown in the table below fo so-called single carbon number slates and the more detailed (but less extended) BTEX component slate. You can find the full list of components in Table 1 in this Excel file.
Table 1 - Example component slates from different PVT laboratories.
Single Carbon Number Group
As we will cover in detail in the sections below, there are many hundreds, if not thousands, of hydrocarbon compounds that make up the composition of petroleum mixtures! In our models and in most PVT reports, these induvidual compounds are usually not given. What is given instead are a set of single carbon number (SCN) components. These are lumped components, also called pseudocomponents, comprised of a large number of "pure" subcomponents. The definition of a SCN component is based on the normal boiling point of the normal paraffin components.
The definition of a SCN component are all the components that have their normal boiling point (Tb) between the noraml boiling point of two neighboring normal paraffin components. The formal definition is given by
where Tb is the boiling point of any given compound in the SCN group, Tb, nCx is the normal boiling point for the normal paraffin component with x carbon atoms. Equation \eqref{eq:scn_definition} is referring to the SCN group referred to as Cx.
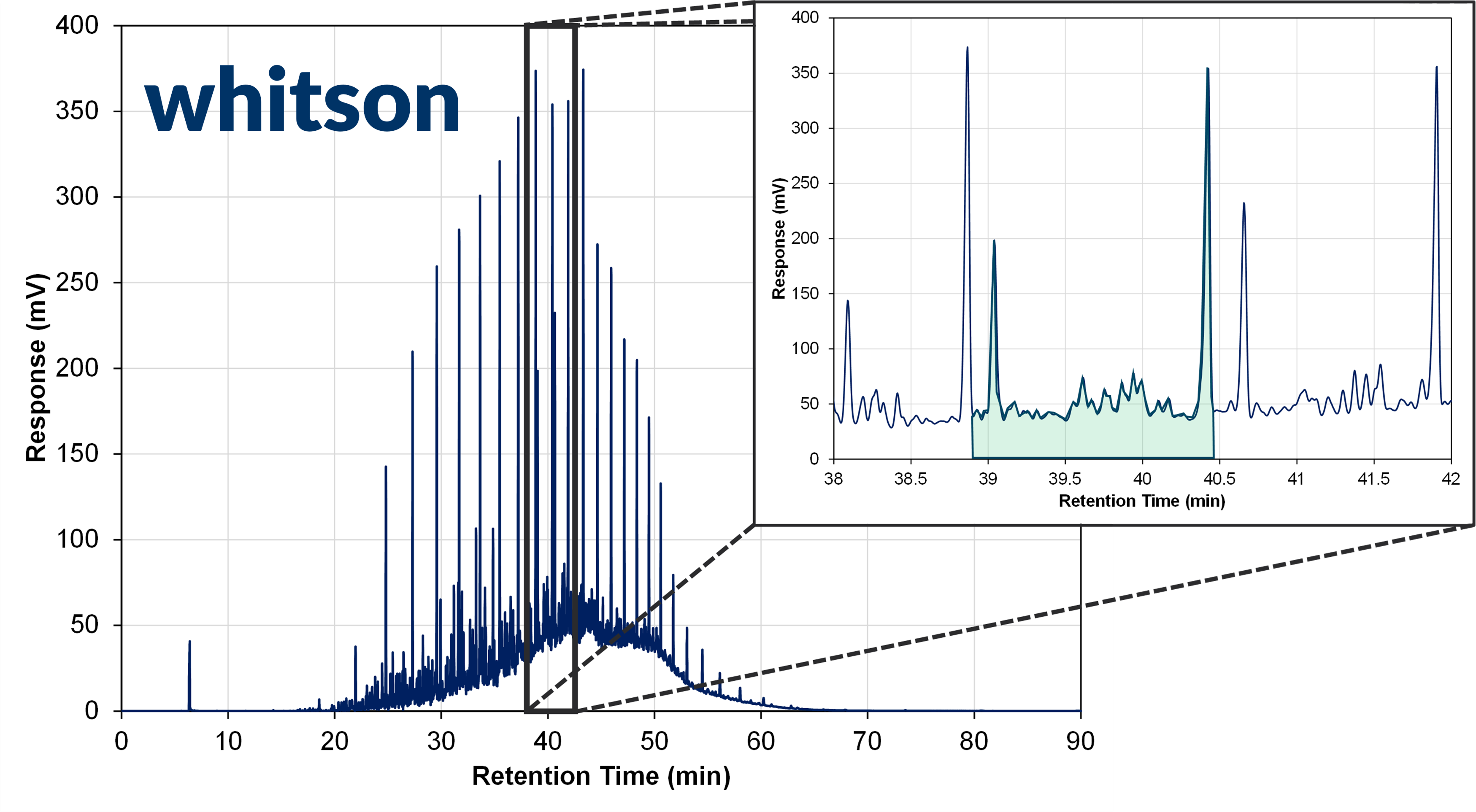
Figure 1 - Example data from a flame ionizing detector (FIT) for a gas chromatograph (GC) of an oil sample. A zoomed image is given at retention times between 38 to 42 minutes, and the GC+FID response for a single carbon number (SCN) component is highlighted in green, with numerous smaller and larger peaks indicating different compounds inside the SCN pseudocomponent. The largest peak at the end of the SCN component's retention time is the normal paraffin peak, and the second largest is most likely the most prominent aromatic component peak.
Basics of Hydrocarbon Compunds
Building an understanding of the different compunds that make up hydrocarbon mixtures, is a bit like building a very uniquely constrained Lego set with primarily only using two Lego pieces (namely the hydrogen and carbon atoms). In the following sections we will take a look at the most common groups of hydrocarbons and what distinguishes them from each other! A set of sources for extracting data for these compounds are also given, as well as a simple Excel database of components and properties that is meant to help build an intuition for what makes different petroleum mixtures behave differently!
Paraffinic Compunds
A paraffinic compound, also called alkanes or saturated hydrocarbons, are hydrocarbon (i.e. composed of hydrogen and carbon) compounds arranges in a tree structure! There is an exponentially increasing number of possible versions / variations / pertubations (also called isomers) of alkanes as you add more carbon and hydrogen atoms! The most common groups of alkanes are the following:
-
Noraml paraffins (also called linear alkanes) - These are linear chains of carbons with hydrogen atoms filling in the available slots.
-
Branched alkanes - These are linear chains of carbon with one or more branching points with hydrogen atoms filling in the available slots.
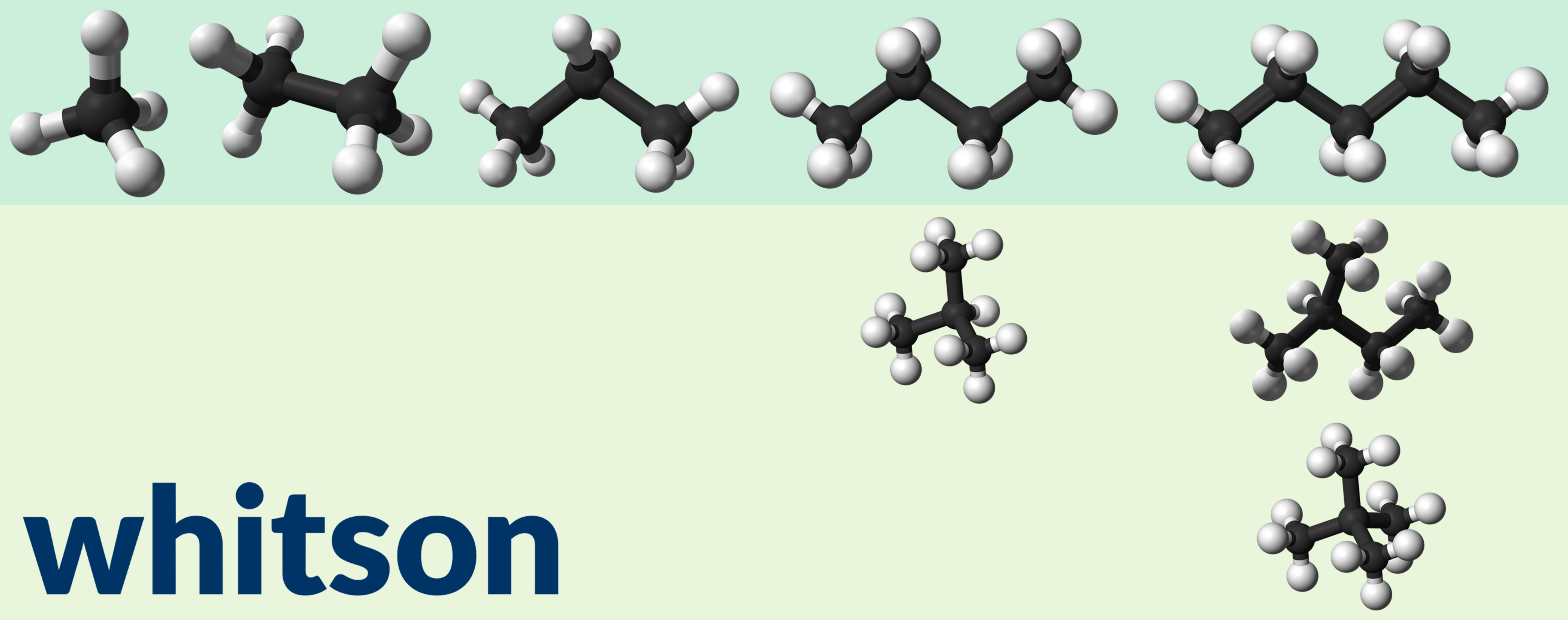
Figure 2 - Ball model examples for linear alkanes (in dark green shaded are) and branched alkanes (light green shaded are) for methane, ethane, propane, butane (normalbutane, and isobutane), and pentane (normalpentane, isopentane, neopentane) from left to right.
The alkanes have the general chemical formula (Hill formula) of CxH2x+2 where x is the number of carbon atoms, and in turn have a molecular weight given by the normal paraffin model.
The Watson characterization factor paraffinic compunds is around 12.
Naphthenes
Naphthenes, also called cycloalkanes, are composite molecules with at least one single bonded cyclic loops of carbon atoms. There are plenty of different variations (isomers) with different alkane branches. Similar to Figure 2, the figure below shows some of the basic cyclic components in relationship to their alkane partners that are in the same single carbon number group (SCN).
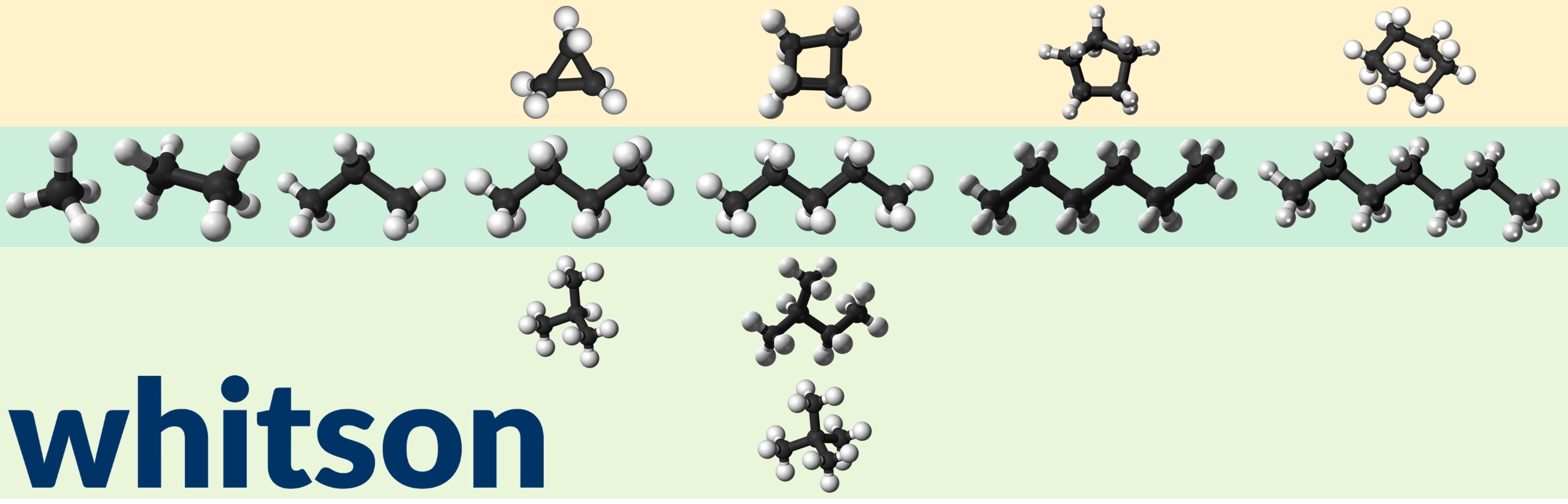
Figure 3 - Ball model examples for linear alkanes (in dark green shaded are), branched alkanes (light green shaded are), and cyclic alcanes for methane, ethane, propane, butane (cyclopropane or C-C3), pentane (cyclobutane or C-C4), hexane (cyclopentane or C-C5), and heptane (cyclohexane or C-C6) from left to right.
The cyclcoalkanes have the general chemical formula (Hill formula) of CxH2x where x is the number of carbon atoms.
The Watson characterization factor paraffinic compunds is around 11.
Aromatic Compounts
Aromatic compunds are composite molecules with at least one benzene ring. The key difference between benzene and cyclohexane is that there are three double bonds in the benzene ring, while cyclohexane ony has single bonds. Benzene is a SCN C7 component, even though it has six carbon atoms, and this is because its normal boiling point is between that of normalhexane (n-C6) and normal heptane (n-C7). Benzene's location relative to its SCN group is shown in Figure 4.
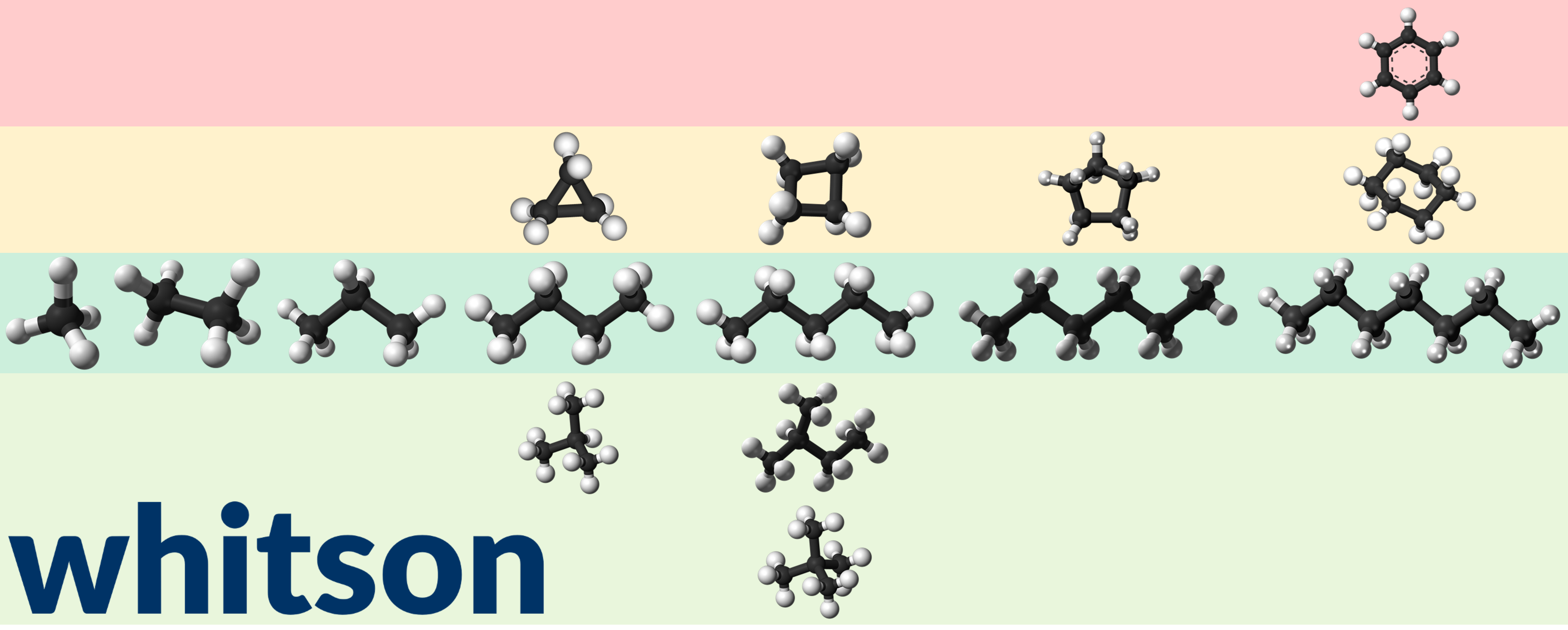
Figure 4 - Ball model examples for linear alkanes (in dark green shaded are), branched alkanes (light green shaded are), cyclic alcanes, and benzene for methane, ethane, propane, butane, pentane, hexane, and heptane (benzene) from left to right.
The Watson characterization factor paraffinic compunds is around 10.
Useful Databases
Below are the links to some useful databases and softwares where you can access thermodynamic properties of different hydrocarbon compunds!
National Institute of Standards and Technology (NIST)
To access the NIST database for thermodynamic properties, use this link, click search, and enter the name of your desired component!
Wolfram Alpha

A lot of useful data can be found on Wolfram Alpha! To find thermodynamic properties here, just go to this link, and type the name of the compound of interest in the search menu. An example of this is shown in the animation above!
Chemo
Chemo is a good search engine for thermodynamic properties and is an aggregate of different databases (like NIST) and group contribution models. If you cannot find the data on Wolfram Alpha or want more details, we would recommend using this search engine! The main drawback is that it usually doesn't have the specific gravirty of compunds!
Excel Example Library
If you want a simple overview of some different groups of C7+ compounds, check out our work-in-progress Excel database here. We'll continue to update and work on this educational database to help bridge the gap between expert chemical engineers and engineers who want to learn!